
Throughout history, medical devices have played a crucial role in improving patient outcomes. From the stethoscope's introduction in the early 19th century to the invention of X-ray machines and pacemakers, these physical devices have completely changed the way healthcare is delivered.
However, in recent years, the rapid evolution of technology has opened up a whole new dimension of possibilities in the healthcare landscape. This progress has significantly impacted how medical professionals work, how individuals manage their health, and how patients and healthcare providers interact. And, at the forefront of this digital revolution lies Software as a Medical Device (SaMD).
In this article, we’ll discuss how SaMD is paving the way for a more patient-centric and technologically advanced future in healthcare, along with its benefits and challenges.
What is SaMD?
According to the International Medical Device Regulators Forum (IMDRF), SaMD is described as "software intended for medical purposes that can fulfill those purposes without being a part of a hardware medical device." In simpler terms, SaMD doesn't need to be integrated into a physical device to serve its intended medical functions. For example, SaMD could function as a standalone application on a mobile phone without any direct connection to a physical medical device.
Analysts project an astounding compound annual growth rate of 21.9 percent for the SaMD market, driving its value to over $86 million by 2027. These figures signify not only innovation but also the possibility of saving lives and transforming healthcare for the better.
To understand this better, take an example of a mobile application that is designed to analyze and interpret electrocardiogram (ECG) data. This SaMD application allows patients to record their ECG using a portable device connected to their smartphone. The application then analyzes the ECG data, identifies irregular heart rhythms, and provides a detailed report to both patients and their healthcare providers.
In this instance, the mobile application acts as a SaMD since it performs essential medical functions (ECG analysis and irregularity detection) independently, without the need for any specialized medical hardware. Patients can conveniently use their smartphones and the associated portable ECG device to monitor their heart health regularly and share vital information with their healthcare team for timely interventions.
On the other hand, the software responsible for running the MRI machine and generating images during an MRI scan is not considered SaMD.
SaMD products should not be confused with fitness apps or software with a medical purpose that is embedded in a medical device(SiMD).
How Can Software Be Classified as a Medical Device (SaMD)?
To be considered SaMD, software must fulfill at least one of the following descriptions:

- Intended for Medical Use: The software must be created and designed to help with medical tasks, such as diagnosing illnesses, providing treatments, or monitoring health conditions.
- Not Necessary for Hardware Medical Devices: Even if SaMD is connected to a hardware medical device, it is still considered SaMD as long as the software itself is not essential for the device to carry out its intended medical functions.
- May Be Used with Other Products: SaMD can be used in combination with other products, including medical devices. For example, it can act as a module or component that enhances the capabilities of a larger medical system.
- Interfaces with Various Devices: SaMD can interact with other medical devices or software, such as hardware medical devices, cell phones, tablets, and even other SaMD software. This versatility allows for seamless data exchange and connectivity within healthcare systems.
Some instances of SaMD comprise:
- Software for analyzing X-ray or CT images in medical imaging
- CAD software that aids in post-processing images to detect breast cancer
- Analytical software for monitoring health metrics (e.g., heart rate or blood glucose)
Examples of products that the FDA doesn't consider Software as a Medical Device (SaMD)
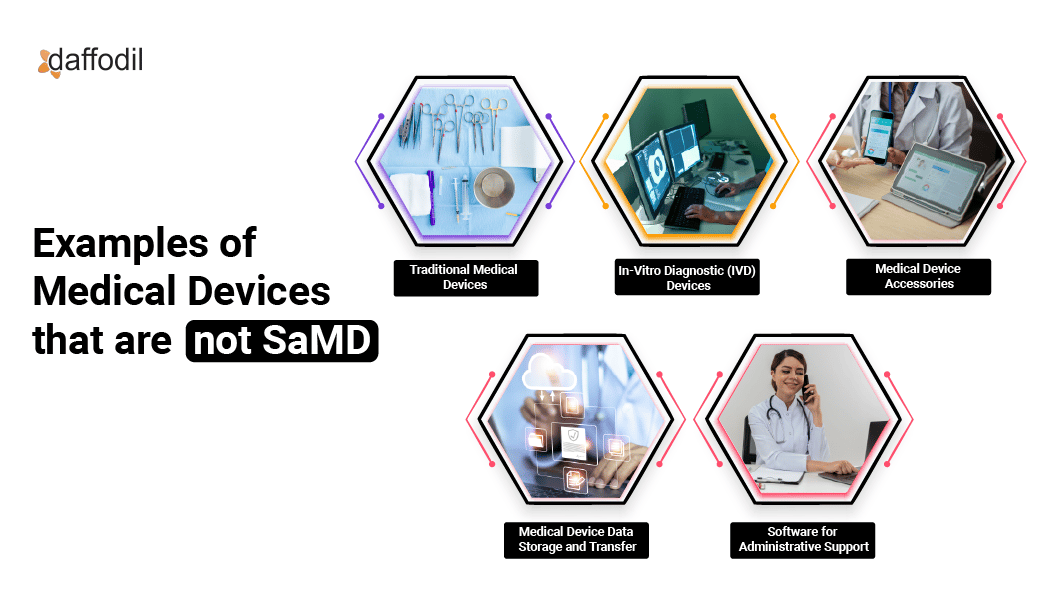
1. Traditional Medical Devices: Devices that are purely hardware-based, such as stethoscopes, surgical instruments, or blood pressure monitors, without any software components.
2. In-Vitro Diagnostic (IVD) Devices: Devices used to analyze specimens outside of the body, such as blood tests or urine tests, that do not involve standalone software for medical purposes.
3. Medical Device Accessories: Accessories that support or enhance the performance of traditional medical devices but are not standalone software applications for medical purposes. Such as a pulse oximeter probe.
4. Medical Device Data Storage and Transfer: Products that facilitate the secure storage and transfer of medical data but do not perform medical functions independently. Such as Hospital information systems(HIS).
5. Software for Administrative Support: Software used for tasks like scheduling, billing, or record-keeping in healthcare settings but not directly involved in medical decision-making.
SaMD should not be confused with SiMD
SaMD and SiMD are two distinct categories of medical software with different roles and regulatory considerations:
| Parameters | Software as a Medical Device | Software in a Medical Device |
| Definition | SaMD refers to standalone software applications designed for medical purposes that can function independently without being part of a physical medical device. | SiMD refers to software that is embedded into a physical medical device and is essential for that device to fulfill its intended medical purpose. |
| Examples | Imaging analytics software, mobile health apps, remote patient monitoring software, and diagnostic software are all examples of SaMD. | Software controlling drug delivery pumps, closed-loop control software in implantable devices, and software in infusion pumps are all examples of SiMD. |
| Role | SaMD performs medical functions directly and can aid in tasks like diagnosing, monitoring, treating, or preventing diseases or medical conditions. | SiMD acts as an accessory to the hardware medical device, enabling and enhancing its functionality. |
| Regulatory Focus | SaMD is regulated based on its potential risks to patients, and its approval is evaluated based on its safety, efficacy, and performance. | The regulation of SiMD is closely tied to the hardware medical device it supports, and its approval is evaluated based on how it influences the safety and effectiveness of the overall device. |
Understanding the distinction between SaMD and SiMD is crucial for medical device manufacturers, regulatory authorities, and healthcare professionals as it determines the regulatory pathway, risk assessment, and compliance requirements for each type of medical software.
Read More: Wearable Technology in Healthcare: How Medical Devices are Enhancing Healthcare Delivery
The Four Risk Levels of SaMDs
SaMDs are categorized into four distinct risk levels, underscoring the significance of developers comprehending their product's placement. These categories are conveniently numbered I to IV, with Category I representing the lowest impact, while Category IV signifies the highest level of risk and consequence.
1. SaMD Category I: This category includes low-risk software intended for general wellness or healthcare-related activities. These products are not designed to make critical medical decisions and generally pose minimal risk to patients and users. Examples of Category I SaMDs are:
- Rehabilitation Monitoring Apps: Apps that collect data such as the ECG rate, heart rate, and walking speed of a rehabilitation patient to track their progress and recovery.
- Asthma Prediction Apps: Software that collects data and anticipates the occurrence of an asthma episode in patients, providing alerts for timely intervention.
- Blood Pressure Tracking Apps: Applications that store historical blood pressure information for later review by healthcare providers.
2. SaMD Category II: Category II SaMDs are designed to support or provide information for diagnostic or screening purposes. While they can assist healthcare professionals in making decisions, they are not standalone diagnostic tools and require clinical oversight. Examples of Category II SaMDs are:
- Diagnostic Decision Support Software: Software that aids healthcare providers in analyzing medical data and providing preliminary diagnostic insights, but the final diagnosis is made by the healthcare professional.
- Radiology Image Analysis Software: Software that assists radiologists in interpreting medical images, providing annotations, and highlighting potential areas of concern.
- Cardiac Rhythm Monitoring Apps: Apps that analyze heart rhythm data collected from wearable devices and provide insights to healthcare professionals for further evaluation.
3. SaMD Category III: Category III SaMDs provide treatment or therapeutic recommendations and can influence medical decisions. They carry a higher risk profile, requiring more extensive clinical validation and regulatory oversight. Examples of Category III SaMDs are:
- Clinical Decision Support Systems: Software that provides treatment recommendations or dosage calculations based on patient data and medical guidelines.
- Digital Therapeutics: Software-based interventions used to treat medical conditions, such as apps providing cognitive behavioral therapy for mental health disorders.
- Personalized Treatment Planning Software: Applications that create tailored treatment plans based on patient-specific data, such as oncology treatment planning software.
4. SaMD Category IV: Category IV SaMDs are intended for real-time critical decision-making, involving monitoring and influencing patient management. These high-risk SaMDs undergo stringent regulatory scrutiny to ensure their safety and effectiveness. Examples of Category IV SaMDs are:
- Clinical Monitoring Systems: Software used to continuously monitor vital signs, such as heart rate and blood pressure, in intensive care units, with the potential for immediate medical interventions.
- Closed-Loop Systems: Such Software automatically adjusts medical device parameters based on real-time patient data, such as insulin pumps for diabetes management.
- Life-Saving Alert Systems: Software that monitors life-critical conditions and triggers alerts in emergencies, such as warning systems for detecting cardiac arrhythmias.
It's essential to understand SaMD classification, as it determines the level of regulatory requirements and the evidence needed to demonstrate the software's safety and effectiveness.
Customer Success Story: Discover how Daffodil drove innovation in healthcare by modernizing a remote patient monitoring app for EKG recording smart devices.
Top 5 Benefits of SaMDs
Enhanced Efficiency: SaMD streamlines healthcare processes, delivering faster and more efficient workflows. By automating tasks and providing quick access to critical information, it enables healthcare professionals to focus on what matters most: delivering top-notch patient care.
Pinpoint Precision: With advanced algorithms and data analysis, SaMD enhances diagnostic accuracy, reducing the risk of human error. Healthcare providers can confidently make well-informed decisions, leading to more precise and effective treatments.
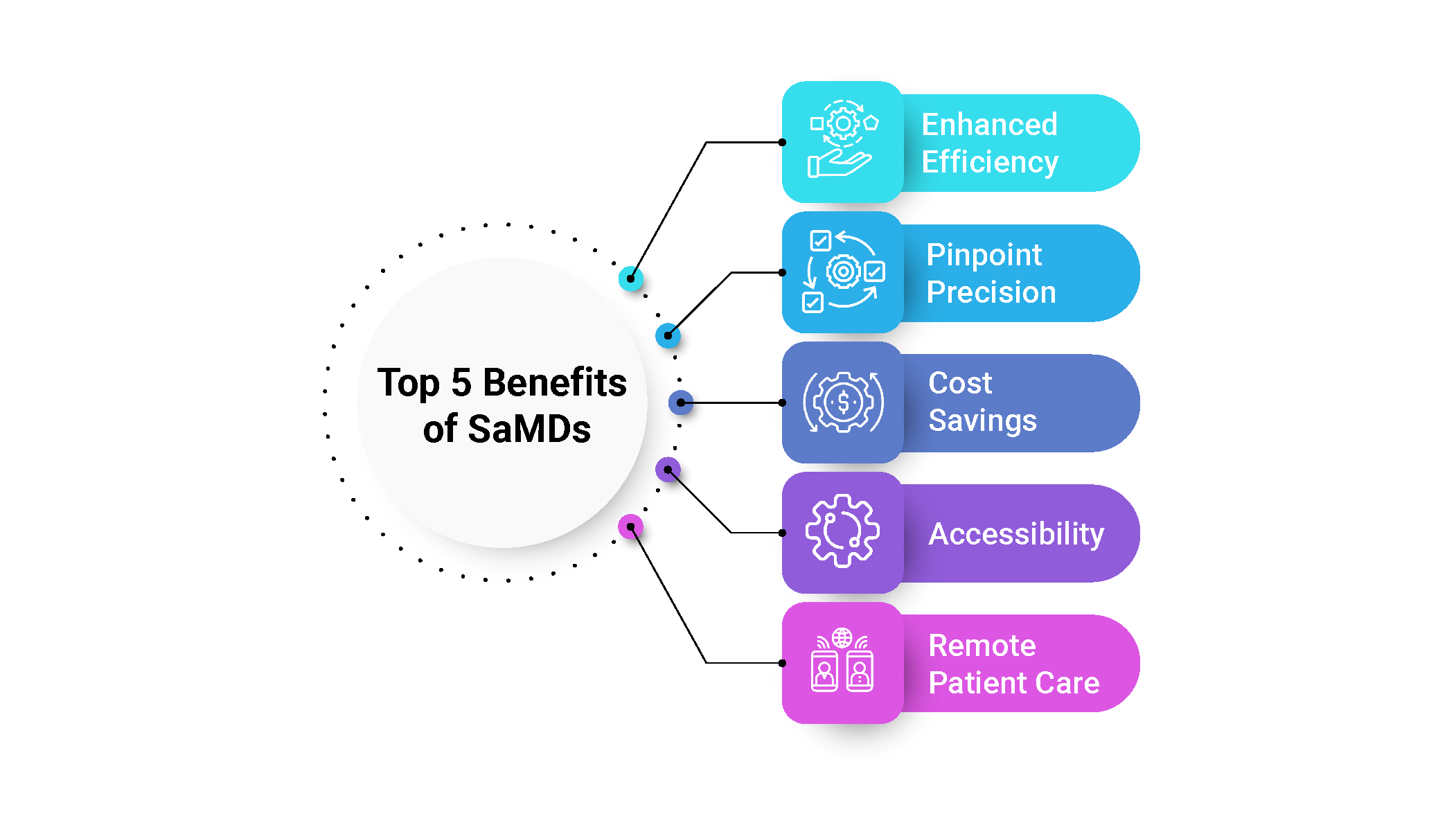
Cost Savings: SaMD optimizes workflows and eliminates manual processes, making healthcare operations more cost-effective. This means healthcare organizations can utilize their resources wisely, saving time and money.
Accessibility: Thanks to SaMD's accessibility on various devices, healthcare professionals can access crucial information on the go. Whether in the hospital, on rounds, or even off-site, timely medical interventions become a breeze.
Remote Patient Care: SaMD enables remote patient monitoring, transforming chronic condition management and post-surgery follow-ups. Patients enjoy greater comfort, while healthcare providers can monitor progress with ease.
4 Key Challenges of Implementing SaMDs
- Regulatory Compliance Complexity: SaMD developers face a complex web of regulations and requirements from health authorities. Meeting these standards demands careful planning and adherence to ensure smooth market entry.
- Security and Privacy Concerns: Protecting patient data is of utmost importance. in 2021 alone, more than 40 million patient records were exposed to online attacks in the U.S. SaMD developers must also ensure compliance with HIPAA standards to protect against the leakage of sensitive information and avoid significant fines and legal consequences faced by healthcare providers.
- Data Accuracy and Reliability: Reliable data is crucial for SaMD's effectiveness, particularly in critical diagnostic or treatment decisions. Developers need to continually fine-tune algorithms and validation methods to ensure accurate and dependable results, especially for high-stakes applications like software products monitoring and analyzing heart rate and blood pressure data in intensive care units.
Any failure in such scenarios could result in severe consequences, potentially leading to fatalities or serious harm to patients or others. Therefore, stringent quality control, continuous testing, and validation are imperative to meet the highest standards of performance and safety. - Inexperienced Developers: Another challenge emerges from the inexperience of many SaMD developers in dealing with the FDA's regulatory procedures. Studies show that bringing a medical device to market can take 3 to 7 years on average. Novice developers might encounter difficulties in obtaining timely product approvals, leading to frustration and potential abandonment of their SaMD projects. Products with higher risk levels to patient health may undergo even more rigorous evaluation processes, further complicating matters.
Is Your Business Prepared for SaMDs?
Though data security and regulations pose challenges, adopting SaMDs presents a strategic opportunity for companies to gain a competitive edge and provide cutting-edge care. As the digital health landscape evolves, investing in SaMDs becomes essential for healthcare enterprises striving to lead the industry toward improved patient outcomes and technological advancement. With SaMDs leading the way, the future of medical device innovation looks promising, presenting endless possibilities to transform and elevate healthcare practices. Adopting this technology can chart a path toward a more efficient and impactful future in the realm of healthcare
Seeking innovative software solutions for better patient outcomes? Check out our healthcare IT services & remote patient monitoring solutions. With years of experience in the healthcare technology domain, our skilled team of developers, designers, and domain experts collaborate closely with clients to understand their unique requirements and challenges. We strive to create personalized SaMD solutions that align perfectly with your goals and vision, ensuring exceptional outcomes for your medical device innovations.





